Following a successful UK primary care study, (funded by SBRI, NHS England) P1vital Ltd has secured €4m in funding from the EU Horizon 2020 SME programme to conduct an international clinical investigation to demonstrate the clinical and cost effectiveness of its lead product, the Predicting Response to Depression Treatment (PReDicT) test, for use in primary care. PReDicT will transform the treatment of depression and benefit patients by improving their health and productivity, healthcare providers through cost savings and the wider economy by reducing absence from work. PReDicT uses a proprietary machine learning algorithm to provide a rapid, objective measure of a patient’s response to an antidepressant which accelerates their recovery from the illness. PReDicT will significantly reduce the burden of depression and the healthcare and societal costs of the illness, estimated globally to be in excess of £550bn.
Why do we need this study?

Depression affects 1 in 10 people and whilst antidepressants are effective in treating depression, the clinical onset of action is slow, taking 4 to 6 weeks before changes in mood become apparent. Furthermore, 2/3 of patients do not respond to the first medication prescribed and must try a series of different drugs, one after the other. During this time a patient’s ability to work and function socially is severely impaired, and individuals may be absent from work for many weeks or months. Indeed, depression is associated with significant socio-economic costs and is predicted to become the greatest cause of disability worldwide by 2030 (World Health Organization, Global Burden of Disease, 2004 update). In 2010, approximately 30 million patients were estimated to have depression in Europe, resulting in aggregated economic costs of approximately €92 billion.
What is the purpose of this study?
The medicines used to treat depression often work slowly and have to be taken for a number of weeks before a patient notices an improvement in their mood. For this reason, the time taken for a primary care physician to optimise effective treatment can be protracted. The objective of the P1vital® PReDicT Test is to improve treatment response and accelerate recovery from depression thereby helping both the patient and the primary care physician and exerting a positive outcome regarding socio-economic benefits.
Benefit to the patients:
- Quicker time to remission and recovery
- Certainty that even if the drug has unpleasant side effects, it will provide benefits and improve mood in the long term
- More informed choice and control for patients and their families
Benefits to physicians:
- Less but more efficient contact time between GP’s and patients
- Ability to make an informed decision about how to proceed with a patient’s treatment
Benefits to economy:
- Lower costs from lost productivity due to sick leave and early retirement
- Reduced burden on the healthcare system through lowering the rate of treatment discontinuation, the cost of testing multiple antidepressants on a patient and the rate of hospitalisations
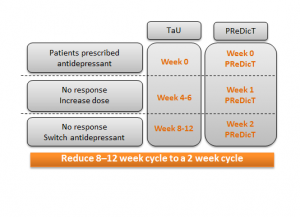
How does the P1vital® PReDicT Test work?
A patient diagnosed with depression will use a computer, tablet or smartphone to complete the P1vital® PReDicT Test before they start their medicine. The test is designed to measure how the prescribed antidepressant changes the way a patient responds to negative emotional bias (thought to be a precursor to mood). The test is repeated after one week. If the test shows the medicine is having no effect, then the GP can change the antidepressant rather than waiting many weeks to see if the patient’s mood is improved or not. In this study we will investigate whether, after 8 weeks of treatment with antidepressants, more patients show an improvement in their depression when the P1vital® PReDicT Test is used to guide treatment versus standard of care.